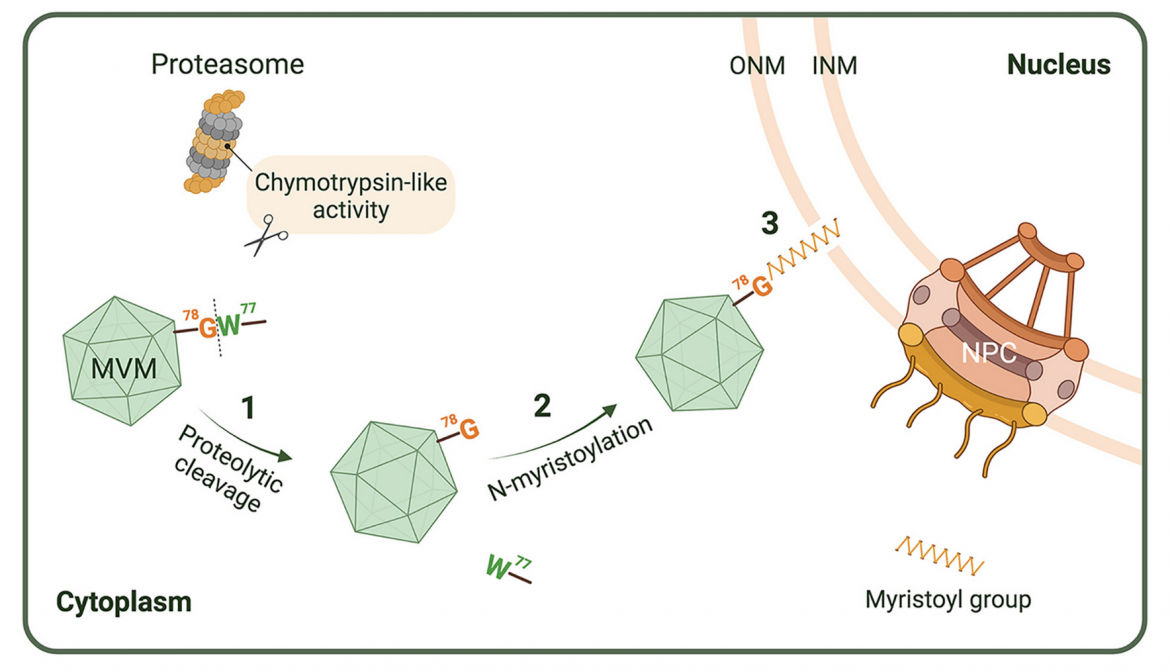
FIG 10. Schematic presentation of the involvement of N-myristoylated VP1u in MVM-induced NE disruption. Step 1: Prior to viral nuclear entry, the MVM capsid interacts with the cellular proteasome at the perinuclear region, whereby VP1u (which has been previously exposed at the virion surface) is cleaved at the C-terminus of W77 by the chymotrypsin-like activity of the proteasome, and subsequently exposes the internal G78 at the N-terminus of the cleaved VP1u. Step 2: Posttranslational N-myristoylation occurs, resulting in the attachment of the myristoyl group to the N-terminal G78. Step 3: N-myristoylated VP1u disrupts the NE in an undefined mechanism, but presumably by insertion of the hydrophobic myristoyl group of VP1u into the lipid bilayers of the nuclear membranes. Diagram created with BioRender.com. Sizes of the NPC and the proteasome compared to the size of MVM have been reduced in the diagram.
ABSTRACT
Being nonpathogenic to humans, rodent parvoviruses (PVs) are naturally oncolytic viruses with great potential as anti-cancer agents. As these viruses replicate in the host cell nucleus, they must gain access to the nucleus during infection. The PV minute virus of mice (MVM) and several other PVs transiently disrupt the nuclear envelope (NE) and enter the nucleus through the resulting breaks. However, the molecular basis of this unique nuclear entry pathway remains uncharacterized. In this study, we used MVM as a model to investigate the molecular mechanism by which PVs induce NE disruption during viral nuclear entry. By combining bioinformatics analyses, metabolic labeling assays, mutagenesis, and pharmacological inhibition, we identified a functional myristoylation site at the sequence 78GGKVGH83 of the unique portion of the capsid protein VP1 (VP1u) of MVM. Performing proteolytic cleavage studies with a peptide containing this myristoylation site or with purified virions, we found tryptophan at position 77 of MVM VP1u is susceptible to chymotrypsin cleavage, implying this cleavage exposes G (glycine) 78 at the N-terminus of VP1u for myristoylation. Subsequent experiments using inhibitors of myristoylation and cellular proteases with MVM-infected cells, or an imaging-based quantitative NE permeabilization assay, further indicate protein myristoylation and a chymotrypsin-like activity are essential for MVM to locally disrupt the NE during viral nuclear entry. We thus propose a model for the nuclear entry of MVM in which NE disruption is mediated by VP1u myristoylation after the intact capsid undergoes proteolytic processing to expose the required N-terminal G for myristoylation.