Graphical abstract
Originally posted as a preprint March 2, 2022
SUMMARY
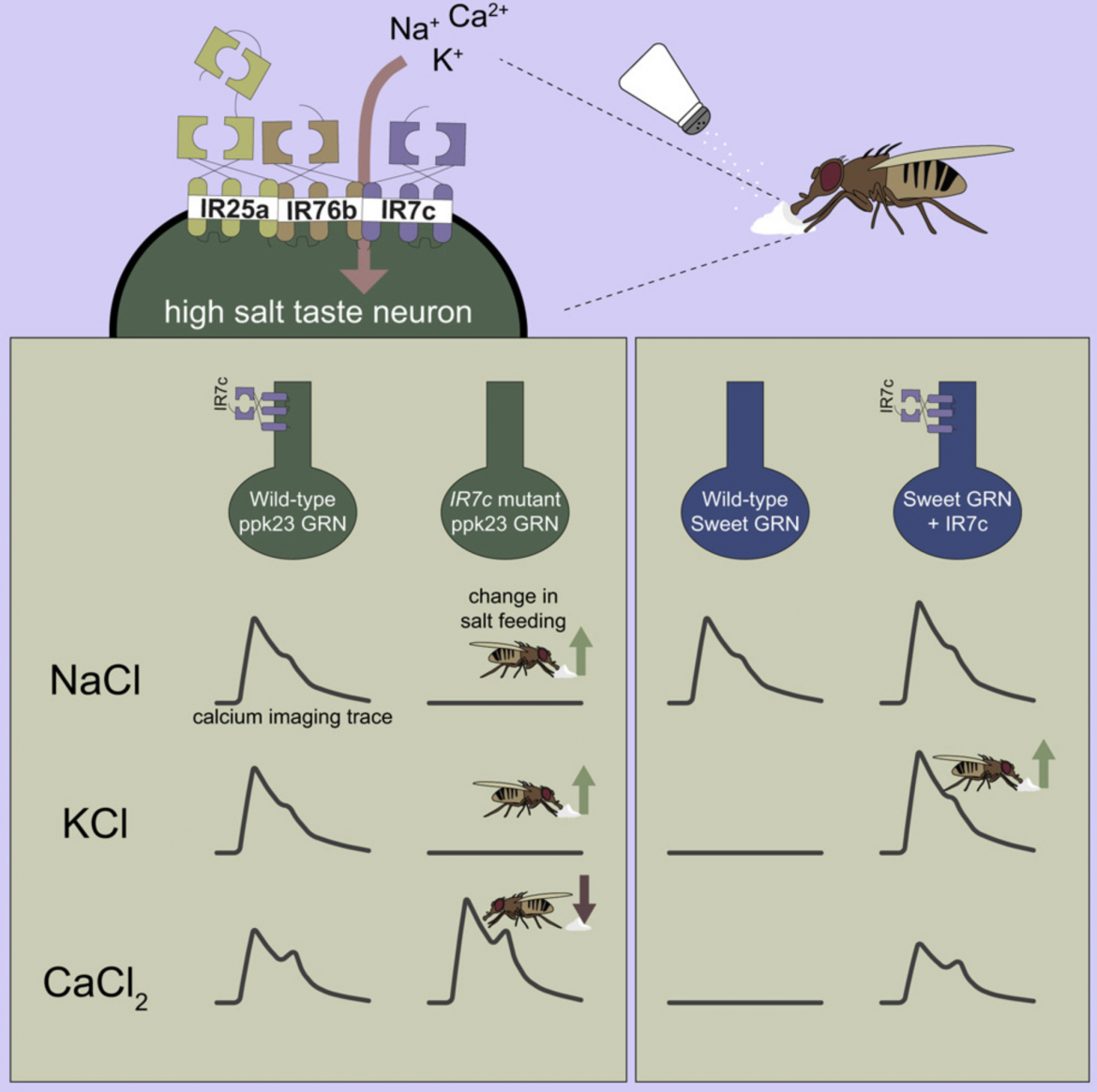
Dietary salt detection and consumption are crucial to maintaining fluid and ionic homeostasis. To optimize salt intake, animals employ salt-dependent activation of multiple taste pathways. Generally, sodium activates attractive taste cells, but attraction is overridden at high salt concentrations by cation non-selective activation of aversive taste cells. In flies, high salt avoidance is driven by both “bitter” taste neurons and a class of glutamatergic “high salt” neurons expressing pickpocket23 (ppk23). Although the cellular basis of salt taste has been described, many of the molecular mechanisms remain elusive. Here, we show that ionotropic receptor 7c (IR7c) is expressed in glutamatergic high salt neurons, where it functions with co-receptors IR76b and IR25a to detect high salt and is essential for monovalent salt taste. Misexpression of IR7c in sweet neurons, which endogenously express IR76b and IR25a, confers responsiveness to non-sodium salts, indicating that IR7c is sufficient to convert a sodium-selective gustatory receptor neuron to a cation non-selective one. Furthermore, the resultant transformation of taste neuron tuning switches potassium chloride from an aversive to an attractive tastant. This research provides insight into the molecular basis of monovalent and divalent salt-taste coding.