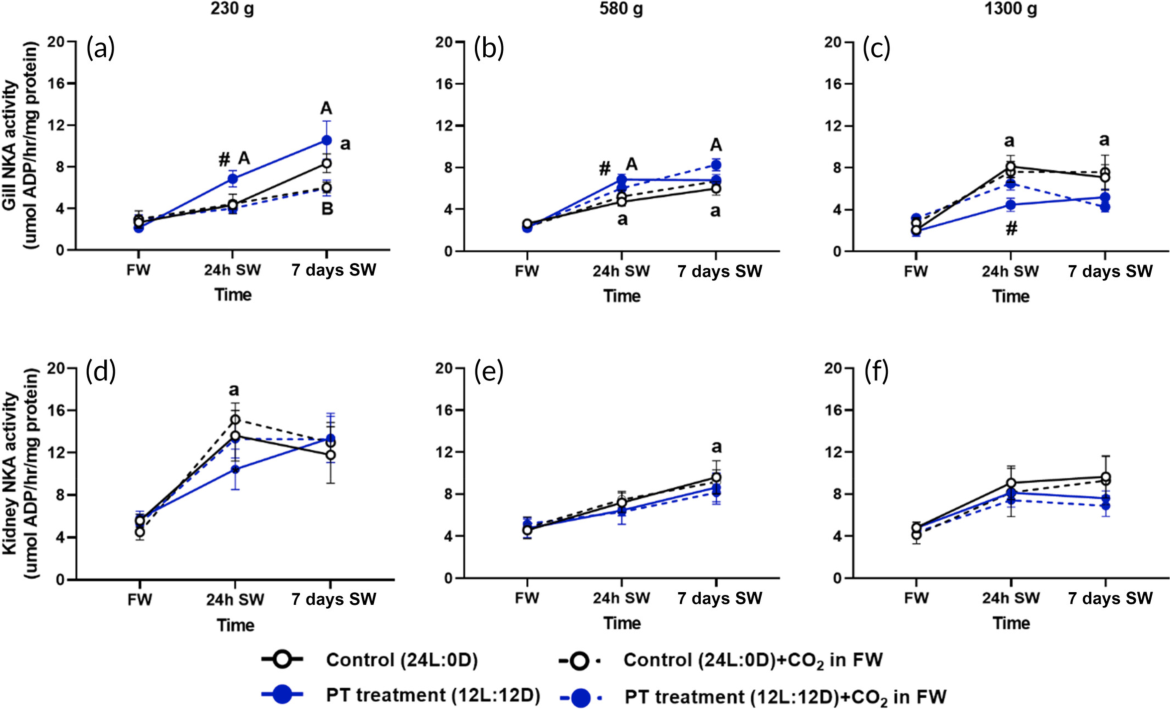
Fig 8 - Na+/K+ ATPase activity (a–c) in gills (gill NKA activity) and (d–f) in kidney (kidney NKA activity) in control (black, constant 24L:0D) and photoperiod (PT) treatment (blue, 8 weeks 12L:12D, followed by 4 weeks 24L:0D before SW [seawater] transfer) in 230-, 580-, and 1300-g fish, respectively. Fish were exposed to 96 h of no added CO2 (solid lines) and 30 mg/L (dashed lines) in fresh water (FW) before transfer to air-equilibrated SW for 24 h and 7 days in SW. Data are shown as mean ± SE; N = 10 per time for 230- and 580-g salmon, and N = 8 per time for 1300-g salmon. Significantly different interactions between time, CO2, and PT are indicated in the figure, and detailed three-way ANOVA outcomes are presented in Table 3. Letters indicate significant differences between SW sampling times compared to FW for control (lowercase a) or PT treatment (uppercase A); significant differences between CO2 and no added CO2 in FW for control (lowercase b) and PT treatment (uppercase B). Black hash signs indicate significant differences between control and treatment at a specific sampling time.
Abstract
There is a growing interest in Atlantic salmon (Salmo salar) aquaculture to extend the time fish are reared in freshwater (FW) recirculating aquaculture systems (RAS), producing larger FW salmon that can then be induced to undergo smoltification before transfer into marine net pens for grow-out and harvest. Smolts can be produced by photoperiod (PT) manipulation in RASs, but little is known about how delaying smoltification to larger body sizes affects susceptibility to elevated CO2 levels (hypercapnia), which can occur at high stocking densities in FW RAS or during transport from FW RAS rearing facilities to marine net pens. To address this, Atlantic salmon were reared from hatch to one of three different sizes (~230, ~580, or ~1300 g) in FW (3 ppt) under continuous light (24:0, light:dark). Once fish reached the desired sizes, a group of salmon were maintained on continuous light 24L:0D to serve as a control salmon. A second group of salmon were exposed to 8 weeks of 12L:12D and then to 4 weeks of 24L:0D to serve as PT treatment salmon, which is the PT manipulation commonly used in Atlantic salmon aquaculture to induce smoltification. At the end of PT manipulation, both control and PT treatment salmon were exposed to 0% or 1.5% CO2 (30 mg/L) for 96 h in FW and then transferred to air-equilibrated seawater (SW, 35 ppt, normocapnia). Salmon were sampled at the end of the 96-h FW CO2 exposure and at 24 h and 7 days in SW for measurements of blood ion/acid–base status, muscle water content (MWC), and gill and kidney Na+/K+ ATPase (NKA) activity. Exposure to 96 h of CO2 in FW resulted in acid–base disturbances in fish from all three size classes, with decreases in blood pH and increases in blood PCO2 and plasma [HCO3−] but no mortality. Despite these large acid–base disturbances in FW, after transfer to normocapnic SW, there were no significant effects of CO2 exposure on extracellular blood pH, intracellular red blood cell pH, or plasma osmoregulatory status for all three sizes of post-smolt salmon. In general, SW transfer was associated with significant increases in plasma ions and osmolality, as well as gill and kidney NKA activity after 24 h and 1 week in SW with no significant impacts between different sizes of salmon. Thus, exposure to 30 mg CO2/L that mimics levels experienced during transport from FW RAS to an SW transfer site may have minimal effects on Atlantic salmon smolts up to 1300 g.